If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, the molarity of the solution is 0.47 M
Step-by-step explanation:
Data given:
mass of sugar = 684.6 grams
molar mass of sucrose = 342.3 grams/mole
volume of water = 4 litres
molarity = ?
molarity is given by the formula =
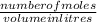 equation 1
equation 1
number of moles =
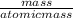 equation 2
equation 2
For number of moles of sucrose equation 2 is used
putting values in it
number of moles =
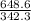
1.89 moles of sucrose are given.
to known the molarity values are put in first equation or formula:
molarity =
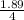
molarity = 0.47 M
thus the molarity of 648.6 grams of sugar in 4 litres of solution is 0.47 M