The answer for the following problem has been mentioned below.
- Therefore the mass of the water is 5.802 grams.
Step-by-step explanation:
Given:
volume of oxygen (V) = 4.50 L
Temperature (T) = 425 K
pressure of oxygen (P) = 2.50 atm
Gram molecular mass of oxygen (M) = 16.0 grams
To calculate:
mass of water (m)
We know;
According to the ideal gas equation;
P × V = n × R × T
As we know;
no of moles =
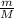
m represents the mass of oxygen (m)
M represents the Gram molecular mass (M)
According to above mentioned equation;
P × V = n × R × T
P represents the pressure of the oxygen
V represents the volume of the oxygen
n represents the no of moles of the oxygen
R represents the universal gas constant
where,
the value of R is 0.0821 L atm/K moles
Substituting the values in the above equation;
2.50 × 4.50 =
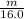 × 0.0821 × 425
× 0.0821 × 425
11.25 =
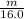 × 34.8925
× 34.8925
180 = m × 34.8925
m =
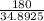
m = 5.158 grams
Therefore the mass of the of oxygen is 5.158 grams
Now;
As we know;
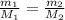
where;
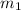 represents the mass of the oxygen
represents the mass of the oxygen
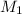 represents the gram molecular mass of the oxygen
represents the gram molecular mass of the oxygen
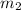 represents the mass of the water
represents the mass of the water
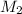 represents the gram molecular mass of water
represents the gram molecular mass of water
From the above given formula,
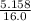 =
=
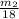
where;
Gram molecular weight of water = 18.0 u
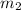 = 5.802 grams
= 5.802 grams
Therefore the mass of the water is 5.802 grams.