Answer:
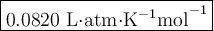
Step-by-step explanation:
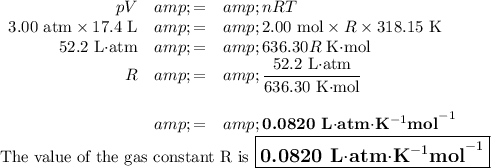
To solve this problem, we can use the Ideal Gas Law:
pV = nRT
Data:
p = 3.00 atm
V = 17.4 L
n = 2.00 mol
T = 45 °C
Calculations:
1. Convert the temperature to kelvins
T = (45 + 273.15) K = 318.15 K
2. Calculate the value of R