The concentration of lead nitrate is 3.48 M.
Step-by-step explanation:
The molarity can be found by dividing moles of sucrose by its volume in litres. We can find the number of moles of sucrose by dividing the given mass by its molar mass. Now we can find the moles as,
Here mass of Pb(NO₃)₂ is 380 g
Molar mass of Pb(NO₃)₂ is 331.2 g/mol
Number of moles =
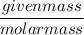
=
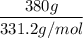
= 1.15 moles
Volume in Litres = 330 ml = 0.33 L
Molarity =
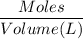
= 3.48 mol/L or 3.48 M
So the concentration of lead nitrate is 3.48 M.