Mass of the water is 2.63 g.
Step-by-step explanation:
Mass of the water, m = ? g
Temperature, ΔT = 15 °C
Heat absorbed, q = 165 J
Specific heat capacity, c = 4.18 J / g °C
q = m × c × ΔT
Now, we have to find the mass of the water by rewriting the above equation as,
m =
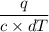
Now Plugin the above values in the equation as,
m =
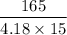
= 2.63 g
So the mass of the water is found as 2.63 g.