0.96 litre will be the new volume of the gas at a pressure of 1.5 atm.
Step-by-step explanation:
Data given:
initial temperature of the gas = 37 degrees
initial pressure P1 = 380 torr or 0.5 atm
initial volume of gas V1= 2.9 litre
final volume V2 = ?
final pressure P2 = 1.5 atm
Assuming that temperature remains constant, the formula used is of Boyle's Law:
P1V1 = P2V2
Rearranging the equation to know the value of final volume of the gas:
V2 =
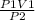
V2 =
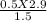
V2 = 0.96 LITRE
the volume of the gas decreases as the pressure of the gas increases.