The resulting pressure will be 2.03 atm.
Step-by-step explanation:
As per the Boyle's law, the volume of a particular mass of a gas is inversely proportional to its pressure applied. As pressure increases, the volume decreases.
P1V1 = P2V2
Here P1 = 1.35 atm
V1 = 5.15 L
P2 = ?
V2 = 3.43 L
The above equation can be rewritten to get P2 as,
P2 =
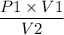
Now, we can plugin the above values as,
P2 =
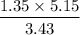
= 2.03 atm
As per the law said, the volume is compressed as the pressure increases.