The mass of a sample of alcohol is found to be = m = 367 g
Hence, it is found out that by raising the temperature of the given product, the mass of alcohol would be 367 g.
Step-by-step explanation:
The Energy of the sample given is q = 4780
We are required to find the mass of alcohol m = ?
Given that,
The specific heat given is represented by = c = 2.4 J/gC
The temperature given is ΔT = 5.43° C
The mass of sample of alcohol can be found as follows,
The formula is c =
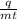
We can drive value of m bu shifting m on the left hand side,
m =
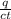
mass of alcohol (m) =
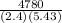
m = 367 g
Therefore, The mass of the given sample of alcohol is
m = 367g
It requires 4780 J of heat to raise the temperature by 5.43 C in the process which yields a mass of 367 g of alcohol.