a) The mass of the excess reactant remaining at completion is 7.36 grams.
b) mass of water produced is 3.51 grams.
c) moles of sodium phosphate produced is 0.0186.
d) grams of sulphur dioxide produced is 12.49 grams.
Step-by-step explanation:
Data given:
mass of sodium sulphite = 20 grams
volume of phosphoric acid = 7 ml
concentration of the phosphoric acid is = 1.83 grams/ml
mass of excess reactant =?
grams of water produced =?
moles of sodium phosphate =?
grams of sulphur dioxide =?
balance equation for the reaction:
2
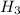 P
P
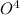 + 3
+ 3
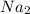
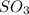 ⇒ 2
⇒ 2
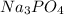 +3
+3
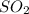 +3
+3
 O
O
Now the number of moles will be calculated by using the formula:
molarity =
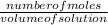
For phosphoric acid number of moles (atomic mass of H3PO4 = 97.99 grams/mole), mass is 1 ml has 1.83 grams so 7 ml wil have 12.81 grams
number of moles =

=
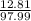
= 0.13 moles of H3PO4 in 7 ml
number of moles of sodium sulphite (atomic mass = 126.04 grams/mole)
=
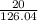
= 0.15 moles
from the stoichiometry:
limiting reagent is the one which yield low amount of product.
3 moles of phosphoric acid gave 2 moles of Na3PO4
0.13 moles give x moles
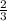 =
=
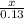
3x = 0.26
x = 0.086 moles of Na3PO4
2 moles of phosphoric acid gave 3 moles of sulphur dioxide and 3 moles of water
0.13 moles of phosphoric acid will give x moles of water and sulphur dioxide.
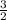 =
=
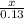 (SO2) and
(SO2) and
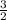 =
=
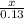
so 0.195 moles of water and 0.195 moles of SO2 is formed.
Now the second reactant:
3 moles of 3
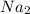
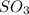 gave 2 moles of Na3PO4
gave 2 moles of Na3PO4
So, 0.15 moles will give
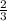 =
=
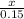
x = 0.1 moles of Na3P04
3 moles
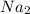
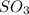 gives 3 mole of water and SO2
gives 3 mole of water and SO2
so 0.15 mole of water and SO2 formed
so the limiting reagent is H3P04
mass of the products:
mass = number of moles x atomic mass
b) mass of water = 0.195 x 18
= 3.51 grams
c) mass of SO2 = 0.195 x 64.06
= 12.49 grams
d ) mass of sodium phosphate = 0.086 x 163.94
= 14.09 grams
a) mass of excess reactant
Sodium sulphite is excess reactant,
so we had started with 0.15 sodium sulphite
ratio of excess and limiting reagent as moles of sodium phosphate from sodium sulphite is 0.1 and from H3P04 is 0.086 moles so excess is
0.1 x 126.04
= 12.64 grams
we started with 20 grams and used 12.64 grams
excess reagent = 20 - 12.64
= 7.36 grams is the excess reagent.