55.83 grams is the mass of Calcium Phosphate will be formed according to the reaction when 75.2 grams of Calcium Sulfate are reacted with an unlimited amount of Sodium Phosphate.
Step-by-step explanation:
Data given:
mass of calcium sulphate reacted= 75.2 grams
atomic mass of calcium sulphate = 136.14 grams/mole
balanced chemical reaction equation:
3CaS
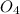 +2
+2
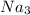 (P
(P
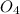 )
)
 ⇒
⇒
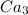 (P
(P
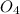 )
)
 + 3
+ 3
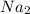 S
S
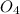
number of moles of calcium phosphate given will be calculated as:
number of moles =

putting the values in the above formula, we get
number of moles =
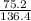
= 0.55 moles of CaS
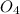 is given
is given
From the balanced equation:
3 moles of CaS
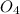 reacts to give 1 mole of calcium phosphate
reacts to give 1 mole of calcium phosphate
so, 0.55 moles of CaS
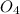 will react to form x moles of calcium phosphate
will react to form x moles of calcium phosphate
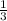 =
=
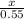
3x = 0.55
x = 0.18 moles of calcium phosphate is formed
mass = number of moles x atomic mass of calcium phosphate
(atomic mass of
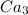 (P
(P
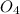 )
)
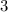 =310.18 grams/mole)
=310.18 grams/mole)
putting the values in the formula:
mass = 0.18 x 310.18
= 55.83 grams of calcium phosphate is formed.