9.32 is the new volume of the balloon.
Step-by-step explanation:
Data given:
Initial volume of the balloon V1 = 10 litres
initial temperature in the room T1 = 24 degrees 0r 297.15 K
final volume V2=?
final temperature T2 = 3.8 degrees OR 276.95
from the values given it is deduced that we have to apply Charles Law
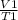 =
=
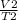
V2 =
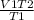
putting the values in the equation:
V2 -
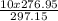
V2 = 9.32 liter
When baloon is taken from a warm room to outside where it is cold the volume of the gas becomes 9.32 litre in the balloon