0.24 moles of Fe2O3 will be produced from 27.0g of Fe assuming O2 is available in excess.
Step-by-step explanation:
Data given:
mass of Fe = 27 grams
mass of oxygen = excess
moles of
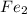
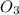 = ?
= ?
Atomic mass of Fe= 55.48 grams/mole
atomic mass of Fe2O3 = 159.69 grams/mole
moles of Fe given =0.48 moles
number of moles =

=
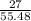
= 0.48 moles
Balanced chemical equation for the reaction is:
4 Fe + 3
 ⇒ 2
⇒ 2
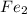
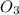
4 moles of Fe gives 2 mole of Fe2O3
So, 0.48 moles will give x mole of Fe2O3
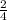 =
=
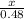
4x = 2 x 0.48
x =
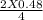
x = 0.24 moles
so 0.24 moles of
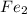
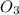 is formed from 27 grams of Fe.
is formed from 27 grams of Fe.