Answer : The oxidation states of chlorine in these three ions
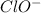 ,
,
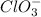 and
and
 is, (+1), (+5) and (-1) respectively.
is, (+1), (+5) and (-1) respectively.
Explanation :
Oxidation number : It represent the number of electrons lost or gained by the atoms of an element in a compound.
Oxidation numbers are generally written with the sign (+) and (-) first and then the magnitude.
When the atoms are present in their elemental state then the oxidation number will be zero.
Rules for Oxidation Numbers :
The oxidation number of a free element is always zero.
The oxidation number of a monatomic ion equals the charge of the ion.
The oxidation number of Hydrogen (H) is +1, but it is -1 in when combined with less electronegative elements.
The oxidation number of oxygen (O) in compounds is usually -2, but it is -1 in peroxides.
The oxidation number of a Group 1 element in a compound is +1.
The oxidation number of a Group 2 element in a compound is +2.
The oxidation number of a Group 17 element in a binary compound is -1.
The sum of the oxidation numbers of all of the atoms in a neutral compound is zero.
The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion.
The given ion is,
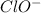
Let the oxidation state of 'Cl' be, 'x'
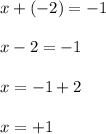
The oxidation number of chlorine in
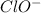 is, (+1)
is, (+1)
The given ion is,
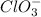
Let the oxidation state of 'Cl' be, 'x'
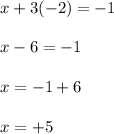
The oxidation number of chlorine in
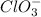 is, (+5)
is, (+5)
The given ion is,

Let the oxidation state of 'Cl' be, 'x'
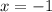
The oxidation number of chlorine in
 is, (-1)
is, (-1)