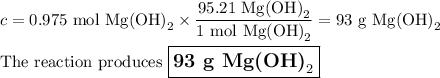Answer:
Step-by-step explanation:
We will need a balanced chemical equation with volumes, molar concentrations. and molar masses.
Mᵣ: 95.21
Mg(OH)₂ + 2HCl ⟶ MgCl₂ + 2H₂O
V/mL: 500 650
c/mol·L⁻¹: 3
1. Moles of HCl
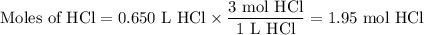
2. Moles of Mg(OH)₂
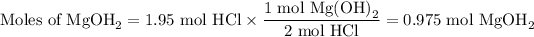
3. Mass of Mg(OH)₂