Answer:
Hope this helps!
Step-by-step explanation:
An atom is the smallest part of an element that maintains the identity of that element.
A molecule is the smallest part of a compund that maintains the identity of that compound. So examples of a molecule are diatomic elements like
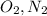
A Bonus Tip: I dont want to confuse you but something I learn that benifited me is to know that molecules are not compounds. For a substance to be considered a compound it has to be more than one atom. In the past, I would confuse molecules as compounds because it would be O
 but its still the same atom so its a molecule not a compound!
but its still the same atom so its a molecule not a compound!