Answer:
At the height of the 176 meter fish's swim bladder will hold the gas without rupturing its swim badder, at this height researchers can safely raise it.
Step-by-step explanation:
Pressure at depth of 500 m =
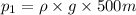
Volume of gas hold by fish's swim bladder =
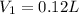
Pressure at dept h where fish's swim bladder can hold maximum gas =
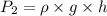
Maximum volume of the gas in fish's swim bladder =
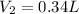
Applying Boyle's law:
 ( at constant temperature)
( at constant temperature)
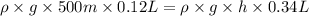
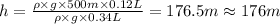
At the height of the 176 meter fish's swim bladder will hold the gas without rupturing its swim badder, at this height researchers can safely raise it.