53.14 grams of Zn
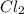 will be produced from 26.0 g of Zn and 42.0 g of HCl.
will be produced from 26.0 g of Zn and 42.0 g of HCl.
Step-by-step explanation:
Balanced chemical reaction:
Zn + 2 HCl ⇒ Zn
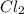 +
+

data given:
mass of Zn = 26 grams
mass of HCl = 42 grams
mass of Zn
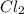 = ?
= ?
here in the reaction Zn is the limiting reagent so mass of
Zn
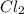 formed will be determined by it.
formed will be determined by it.
atomic mass of zinc =65.38 grams/mole
number of moles =

putting the values in the above equation:
number of moles of zinc =
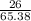
number of moles of zinc = 0.39 moles
From the balanced reaction it is seen that
1 mole of Zn produced 1 mole of Zn
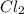
0.39 moles will produce x moles of Zn
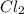
0.39 moles of Zn
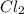 (atomic mass of Zn = 65.38 grams/mole)
(atomic mass of Zn = 65.38 grams/mole)
mass = atomic mass x number of moles
= 0.39 x 136.28
= 53.14 grams
53.14 grams of Zn
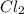 is formed
is formed