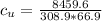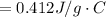Answer:
the specific heat of the unknown compound is
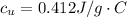
Step-by-step explanation:
Generally the change in temperature of water is evaluated as
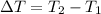
Substituting 16.1°C for
 and 27.4°C for
and 27.4°C for

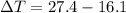
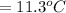
Generally the change in temperature of unknown compound is evaluated as
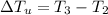
Substituting 27.4°C for
 and 94.3°C for
and 94.3°C for
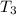
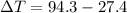
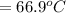
Since there is an increase in temperature then heat is gained by water and this can be evaluated as
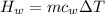
Substituting 179.1 g for m , 4.18 J/g.C for
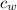 (specific heat of water)
(specific heat of water)
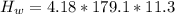
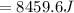
Since there is a decrease in temperature then heat is lost by unknown compound and this can be evaluated as
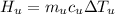
By conservation of energy law
Heat lost = Heat gained
Substituting 306.9 g for
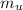 , 8459.6J for
, 8459.6J for
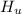
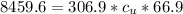
Therefore