Answer:
Option d.
Step-by-step explanation:
The activation energy is the minimum amount of energy that must be given to the reactants of a system for the chemical reaction to proceed to the formation of products. The activation energy is related to the amount of energy that the molecules must have to react when they collide.
The molecules must have the energy to be able to react and form the called activation complex or the transition state of the reaction. This does not long enough to be detected as the reaction intermediate.
The Arrhenius equation gives the relationship between the activation energy and the rate of the reaction.
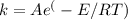
Where:
A: is the pre-exponential factor for the reaction
R: is the universal gas constant
T: is the temperature
k: is the reaction rate
Higher the activation energy the slower the reaction, and vice versa, the lower the activation energy the fastest the reaction.
Hence, the statement that expresses the correct sequence of a chemical reaction that has a low activation energy is the option d: reactants collide with reactants to form an activated complex that separates into products.
I hope it helps you!