Answer:
0.200 L
Step-by-step explanation:
To understand these exercises, one has to know the variables we are working with, what we are looking for, and also what equation to use. The variables we have are the following:
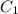 = 10.0 M
= 10.0 M
 = 4.00 L = 4000 mL
= 4.00 L = 4000 mL
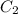 = 0.500 M
= 0.500 M
The subscript 1 belongs to the first solution and the subscript 2 belongs to the second solution. C = Concentration and V = Volume
We have to find out the volume needed of the solution 1 to prepare solution 2, so:
 = ?
= ?
We know that we will have the same amount of mols in solution 1 and solution 2:
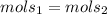
We can infere that mols = V x C (only in M), so:
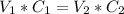 ⇒ We have to solve for V1 ⇒
⇒ We have to solve for V1 ⇒
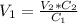
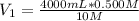 ⇒
⇒
 = 200 mL = 0.200 L
= 200 mL = 0.200 L
The most correct answer is 0.200 L (Note: Always have in mind the significant figures, which in this case is three sig. figs. Don’t lose points in something preventable!)