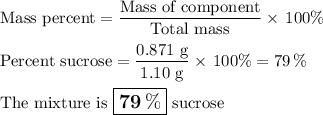Answer:
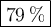
Step-by-step explanation:
I assume the volume is 2.50 L. A volume of 25.0 L gives an impossible answer.
We have two conditions:
(1) Mass of glucose + mass of sucrose = 1.10 g
(2) Osmotic pressure of glucose + osmotic pressure of sucrose = 3.78 atm
Let g = mass of glucose
and s = mass of sucrose. Then
g/180.16 = moles of glucose, and
s/342.30 = moles of sucrose. Also,
g/(180.16×2.50) = g/450.4 = molar concentration of glucose. and
s/(342.30×2.50) = s/855.8 = molar concentration of sucrose.
1. Set up the osmotic pressure condition
Π = cRT, so
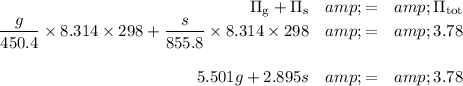
Now we can write the two simultaneous equations and solve for the masses.
2. Calculate the masses
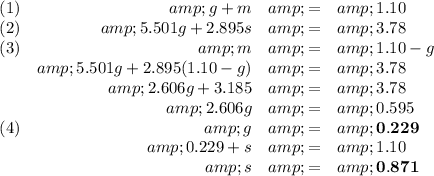
We have 0.229 g of glucose and 0.871 g of sucrose.
3. Calculate the mass percent of sucrose