1. 842g of NaOH will form 547.3 g of Al(OH)₃
2. The yield is 93.55%
Step-by-step explanation:
3NaOH + Al → Al(OH)₃ + 3Na
1.
Molar mass of NaOH = 40 g/mol
Molar mass of Al = 27 g/mol
Molar mass of Al(OH)₃ = 78 g/mol
According to the balanced equation:
3 moles of NaOH requires 1 mole of Al to form 1 mole of Al(OH)₃
The ratio of NaOH : Al : Al(OH)₃ = 3 : 1 : 1
3 X 40 g of NaOH reacts with 27 g of Al to form 78 g of Al(OH)₃
120 g of NaOH + 27g of Al → 78 g of Al(OH)₃
120g of NaOH form 78g of Al(OH)₃
1g of NaOH will form
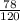 g of Al(OH)₃
g of Al(OH)₃
842g of NaOH will form
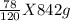 of Al(OH)₃
of Al(OH)₃
= 547.3 g of Al(OH)₃
Therefore, 842g of NaOH will form 547.3 g of Al(OH)₃
2. Only 512 g of Al(OH)₃ is formed
Yield % = ?
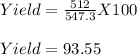
Therefore, the yield is 93.55%