548.55 grams of aluminum hydroxide should theoretically form.
Step-by-step explanation:
Balanced equation for the reaction:
3 NaOH + Al ⇒ Al(OH)3 +3 Na
DATA GIVEN:
mass of NaOH = 842 grams, atomic mass =39.9 grams/mole
mass of Al = 750 grams, atomic mass = 26.9 grams/mole
aluminum hydroxide theoretical yield = ?
Moles of NaOH reacted
number of moles =

putting the values in the equation
NaOH =
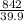
= 21.1 MOLES OF NaOH
Al =
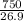
= 27.8 moles
from the equation
from 3 moles of NaOH 1 mole of Al(OH)3 is produced
21.1 moles of NaOH will react to give x moles of Al(OH)3
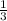 =
=
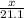
7.03 moles of Al(OH)3 is formed.
and
1 mole of Al(OH)3 is formed from 1 mole of Al in the reaction
so, 27.8 Moles will react to give give 27.8 moles of Al(OH)3 limiting reagent of the given reaction is NaOH
mass of Al(OH)3 =7.03 x 78 (atomic mass of Al(OH)3)
= 548.55 grams
theoretical yield from the given data is 548.55 grams