Answer:
The piston will move down
Step-by-step explanation:
We can solve this problem by using Charle's Law, which states that:
"For a fixed mass of an ideal gas kept at constant pressure, the volume of the gas is directly proportional to the absolute temperature of the gas"
Mathematically:
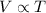
where
V is the volume of the gas
T is the absolute temperature of the gas
In this problem, we have a gas placed in a container with a moveable piston. We are also told that the gas is kept at constant pressure, so we can apply Charle's Law.
We are told that the gas is cooled down: this means that its absolute temperature decreases. Therefore, according to Charle's Law, this means that the volume of the gas also decreases: therefore, the piston of the container will move down.