Answer:
n=28.03
Step-by-step explanation:
For this problem you would have to use the Ideal gas law formula which is
 . In this formula, R is called the ideal gas law constant which is R= 0.08205
. In this formula, R is called the ideal gas law constant which is R= 0.08205
For your problem, this formula would look like this...
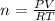
First you would have to convert Celsius to Kelvin: 273 + 127 C= 400K
Your problem would look like this in the formula
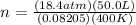
Once solved n= 28.03 mol