Answer : The correct option is, (C) limiting reagent
Explanation :
Excess reagent : It is defined as the reactants not completely used up in the reaction.
Limiting reagent : It is defined as the reactants completely used up in the reaction.
Theoretical yield : It is calculated from the amount of the limiting reagent present in the reaction.
Actual yield : It is experimentally determined.
Percent yield : It is the percent ratio of actual yield to the theoretical yield.
The balanced chemical reaction will be:
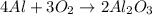
In this reaction, aluminium is a limiting reagent that limits the formation of product and the oxygen is an excess reagent.
Hence, the 0.191 g solid aluminum is the limiting reagent.