Answer:
74.47 kPa
Step-by-step explanation:
As provided in the question, Boyle's law follows that
Pressure 1 x volume 1=pressure 2 x volume 2
Given that
Pressure 1 =79.06 kPa
volume 1=63.299L
pressure 2=unknown
Volume 2=69.057L
Substituting these into the formula then
79.06*63.299=69.057*P2
Making pressure 2 the subject of the formula
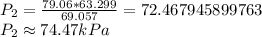
Therefore, the resulting pressure, rounded off to two decimal places is approximately 74.47 kPa