The pressure needed to change the volume to 24 L is 0.796 atm.
Step-by-step explanation:
It is known by Boyle's law that the pressure experienced by the gas molecules will be inversely proportional to the volume occupied by the molecules.
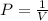
So as the initial volume is said to be 22.4 L, consider it as V₁ = 22.4 L. Then the initial pressure is said to be 0.853 atm, so P₁ = 0.853 atm. So we have to determine the new pressure P₂ when the volume is changed to V₂ = 24 L. As there is increase in the volume, the pressure should be decreased due to Boyle's law. Thus, as per Boyle's law, the two pressures and their volumes can be related as
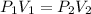
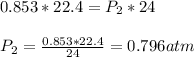
Thus, the pressure gets decreased to 0.796 atm on increase in the volume to 24 L.
So the pressure needed to change the volume to 24 L is 0.796 atm.