1373 atm is the pressure exerted by the gas if the container were transported across the desert at 40.0°C.
Step-by-step explanation:
Data given about steel oxygen cylinder:
Initial temperature T1 = 25 Degrees or 273.15+25 = 293.15 k
initial pressure on the gas P1 = 1250 atm
Final temperature T2 = 40 degrees or 40 + 273.15 = 322.15 K
final pressure P2 =?
To calculate the final pressure, the equation used is of pressure law:
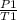 =
=
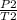
Putting the given values in the equation:
P2 =
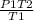
P2 =
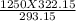
P2 = 1373 atm
The pressure exerted by the oxygen gas when transported across the desert as asked in the question is 1373 atm.