1) Mass:
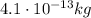
2) Electrons: 125
Step-by-step explanation:
1)
The electric force exerted on the oild drop is given by
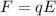
where
q is the charge on the oil drop
E is the magnitude of the electric field
The electric field between two parallel plates can be written as
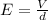
where
V is the potential difference
d is the separation between the plates
So the electric force is
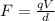 (1)
(1)
On the other hand, the gravitational force on the oil drop is
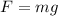 (2)
(2)
where
m is the mass of the drop
g is the acceleration due to gravity
The two forces have opposite directions (electric force: upward, gravity: downward), so the oil drop remains in equilibrium if the two forces have same magnitude. So,
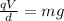
Here we have
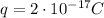 is the charge of the oil drop
is the charge of the oil drop
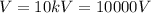 is the potential difference
is the potential difference
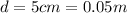 is the separation between the plates
is the separation between the plates
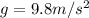 is the acceleration due to gravity
is the acceleration due to gravity
Solving for m, we find the mass of the oil drop:
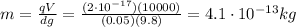
2)
From the text of the problem, we know that the net charge on the oil drop is
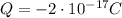
Where the charge is negative since it is due to an excess of electrons (which are negatively charged).
The net charge on the oil drop can be written as
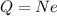
where
N is the number of excess electrons
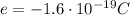 is the charge on one electron (the fundamental charge)
is the charge on one electron (the fundamental charge)
Therefore, here we can solve the formula for N, to find the number of excess electrons on the oil drop:
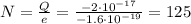
So, 125 excess electrons.