Oxygen is the limiting reagent.
Step-by-step explanation:
2H₂ + O₂ → 2H₂O
Molar mass of H₂ = 2 g
Molar mass of O₂ = 32g
According to the balanced equation:
2 X 2g of H₂ reacts with 32 g of O₂
4g of H₂ reacts with 32g of O₂
1g of H₂ will react with
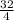 = 8g of O₂
= 8g of O₂
So, 16.7g of H₂ will react with
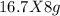 = 133.6g of O₂
= 133.6g of O₂
However, O₂ is just 15.4g
Since O₂ is present in less quantity, O₂ is the limiting reagent.