3.84 grams is the mass of calcium used in 3 moles of Ca(OH)2 so that it contains the same mass of calcium as the first sample.
Step-by-step explanation:
Data given:
first sample:
10 grams of Ca3(PO4)2
second sample :
mass of Ca(OH)2 =?
mass of the calcium should be same in both the solutions
In Ca3(PO4)2, 3 moles of calcium is present so
mass = 3 x 40.07 ggrams/mole
mass of calcium= 120.21 grams in Ca3(PO4)2
In Ca(OH)2 only 1 mole of calcium is present so to equal the mass 3 moles should be taken
3 moles of Ca(OH)2 is taken
number of moles of calcium in grams of Ca3(PO4)2
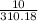
= 0.032 moles.
1 mole of Ca3(PO4)2 has 3 moles of calcium
0.032 moles of Ca3(PO4)2 has x moles
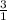 =
=
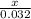
= 0.096 moles
so 0.096 moles or 3.84grams of calcium will be used to make solutions of
Ca3(PO4)2 and Ca(OH)2 separately of 10 grams each.