We need to add 2 liters of water !
Step-by-step explanation:
Here we have , I have 4.0 L of a 3.0 M NaCl solution and I want to dilute it to 2.0 M. We need to find How much water do I need to add. Let's find out:
4.0 L of a 3.0 M NaCl solution , i.e.
⇒ Molarity = ( Number of moles ) / ( Volume of solution in L )
⇒
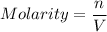
⇒
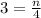
⇒

Now , Molarity is changed to 2M i.e.
⇒
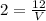
⇒
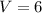
So , change in volume is :
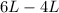 =
=
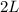
Therefore , We need to add 2 liters of water !