Answer:
The concentration of the acid HA is 0.1727 M.
Step-by-step explanation:
The formula for the relationship between concentration and volume is:
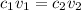 where 1 and 2 are the values for acid and base respectively.
where 1 and 2 are the values for acid and base respectively.
Given:
c1 = ?
v1 = final vol - initial vol = 22.50 - 0.55 mL = 21.95 mL
c2 = 0.1517 M
v2 = 25.o mL
∴ c1 =
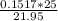
concn = 0.1727 M