Answer:
1. V=19.3 ml
2. mass=74.8g
3. molarity of dilted solution=0.463M
Step-by-step explanation:
1.
First calculate the mole ,
given mass=53g
molecular weight=98g/mole
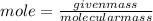
mole=0.54
molarity=28M
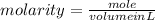
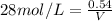
V=19.285ml
V=19.3 ml
2.
First calculate the mole of calcium hydroxide,
molariy=1.9M
volume=532 ml=0.532L
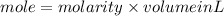
mole=1.01 mol;
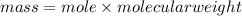
molecular weight of calcium hydroxide= 74g/mol
mass=74.8g
3.
First calculate the mole before adding water
molariy=0.505M
initial volume=124 ml=0.124L
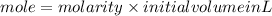
mole=0.063 mol;
after dilution volume=136 ml =0.136L
quantity of solute remains unchanged during dilution of solution.
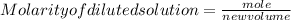
molarity of dilted solution=0.463M