16.4g of solute is present in 500mL of the solution.
Step-by-step explanation:
Given:
Volume of solution, V = 500mL = 0.5L
Molarity of Sodium phosphate, M = 0.2M
Molecular weight of Na₃PO₄ = 164 g/mol
Mass of the solute, m = ?
We know:
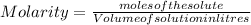
And
Number of moles =
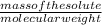
On substituting the value we get:
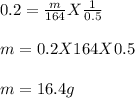
Therefore, 16.4g of solute is present in 500mL of the solution.