This is an incomplete question, here is a complete question.
Consider the reaction between nitrogen and oxygen gas from dinitrogen monoxide.
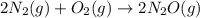
Given: delta Hrxn= +163.2kJ
Calculate the entropy change in the surroundings when this reaction occurs at 25 degrees C.
Answer : The entropy change in the surroundings is, -547.6 J/K
Explanation :
Formula used to calculate the entropy change in the surroundings is:
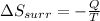
where,
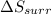 = entropy change in the surrounding
= entropy change in the surrounding
Q = heat energy
T = temperature =
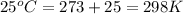
Given:
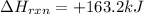
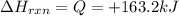
Now put all the given values in the above formula, we get:
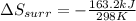
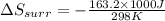

Therefore, the entropy change in the surroundings is, -547.6 J/K