Answer:
pH = 10.505
Step-by-step explanation:
Molar mass of Amphetamine ( C9H13N) = 135 g/mol
Given that the concentration of Amphetamine = 225 mg/L
mass of Amphetamine in one Liter =
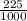 = 0.225 g
= 0.225 g
Number of moles of Amphetamine in one liter =
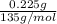
= 0.001667 mol
∴ molarity = 0.0017 M
C₉H₁₃N + H₂O --------> C₉H₁₃NH⁺ + OH⁻
I(M) 0.001667 M 0 0
C(M) -x x x
E(M) 0.001667 - x x x
Pkb = -log Kb = 4.2
∴ Kb = 6.309 x 10⁻⁵
Kb = 6.309 x 10⁻⁵
Equilibrium constant = [C₉H₁₃NH⁺][OH⁻]/ [C₉H₁₃N]
6.309 x 10⁻⁵ = x² / 0.001667-x
where 0.001667 -x ≅ 0.001667
Then;
x² = 6.309 x 10⁻⁵ × 0.001667
x² = 1.0517103 × 10⁻⁷
x =
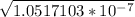
x = 0.00032 M
x = [OH-] = 0.00032 M
∴ pOH = -log [OH-]
pOH = -log (0.00032)
pOH =3.495
pH = 14 - 3.495
= 10.505