Answer:
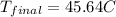 =final temperature
=final temperature
Step-by-step explanation:
In the question specific heat of water is not given but we should know the value of that and it 4.18Jg∘C
Specific heat means how much heat is required to increase the temperature of 1 gram of substance that substance by 1∘C .
Equation between heat lost or gain and the change in temperature.
q=m⋅c⋅ΔT , where
q - the amount of heat
m - the mass of the sample
c - specific heat of sample
ΔT - change in temperature
put all the given value into this ,
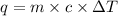
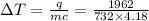
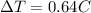
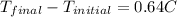
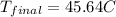 =final temperature.
=final temperature.