Answer:
The volume of 6 M NaOH is 5.5 ml
Step-by-step explanation:
The equation of reaction is
C₃H₅(C₁₇H₃₅COO)₃+3 NaOH → C₃H₅(OH)₃+3 C₁₇H₃₅COONa
The molar mass of triglyceride M is 890 g/mole, so in 10.0 g of its are
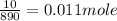
From the equation of reaction one mole of triglyceride reacts with three moles of NaOH.
So,
the quantity moles of NaOH which actually needed to react is:
0.011 x 3=0.033 moles.
These quantity moles of NaOH are consist in
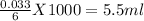
∴The volume of 6 M NaOH is 5.5 ml