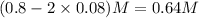Answer: Concentration of
 at equilibrium = 0.08 M
at equilibrium = 0.08 M
Concentration of
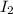 at equilibrium =0.08 M
at equilibrium =0.08 M
Concentration of
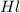 = 0.64M
= 0.64M
Step-by-step explanation:
Moles of
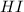 = 4.00 mole
= 4.00 mole
Volume of solution = 5.00 L
Initial concentration of
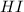 =
=
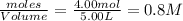
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as

For the given chemical reaction:
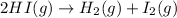
Initial conc. 0.8 M 0 M 0 M
At eqm. conc. (0.8-2x) M (x) M (x) M
The expression for
 is written as:
is written as:
![K_c=([H_2]^1[I_2]^1)/([HI]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/lgqf25fl0xi7e6w6lebvwb6r6rmeljhmv6.png)
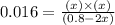
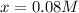
Concentration of
 at equilibrium = x M = 0.08 M
at equilibrium = x M = 0.08 M
Concentration of
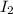 at equilibrium = x M = 0.08 M
at equilibrium = x M = 0.08 M
Concentration of
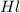 = (0.8-2x) =
= (0.8-2x) =