Answer:
Mass of oxygen are formed is = 1.808 gm
Step-by-step explanation:
Reaction equation of given condition
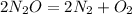
No. of moles of
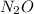
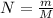
m = 5 gm & M = 44 gm
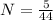
N = 0.113 mol
Now no. of moles of oxygen formed =
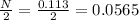
Now mass of oxygen formed is
m = N M
m = 0.0565 × 32
m = 1.808 gm
Thus mass of oxygen are formed is = 1.808 gm