Answer:
The pH of the buffer is 3.54.
Step-by-step explanation:
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([NaHCO_3])/([H_2CO_3]))](https://img.qammunity.org/2021/formulas/chemistry/college/u4vcpvvdkza4axck6mf6pbrc6x9vqu19yq.png)
We are given:
 = negative logarithm of acid dissociation constant of carbonic acid
= negative logarithm of acid dissociation constant of carbonic acid
![pK_a=-\log[K_a]=-\[1.8* 10^(-5)]=4.74](https://img.qammunity.org/2021/formulas/chemistry/high-school/9nsf0ivsyrrv43wl385z6jy38l8tmiirec.png)
![[NaC_2H_3O_2]=0.059 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/itvib56t835skwecpdlw4k3nvlti71zu33.png)
![[HC_2H_3O_2]=0.94 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/j2e4vx3uhs3br5pcuhlkvdgqzoqyhrpk8f.png)
pH = ?
Putting values in above equation, we get:
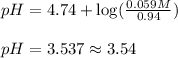
The pH of the buffer is 3.54.