The energy absorbed by iron block is 444 J and the energy absorbed by copper block is 386 J.
Step-by-step explanation:
As the values given in the problems are of mass of the objects which is 10 g, the temperature is changed from 300 K to 400 K. Since the mass and temperature change in two objects that is iron and copper are kept constant, then the heat energy absorbed by them will differ based on their specific heat capacity of the materials of the block.
So, the specific heat capacity of copper is 0.386 J/g°C and the specific heat capacity of iron is 0.444 J/g°C.
So, in order to determine the energy absorbed by iron block, we need the variables m = 10 g, T₂ = 400 K = 400-273.15 = 126.85 °C and T₁ = 300 K = 300-273.15 = 26.85 °C and c = 0.444 J/g°C.
The formula is
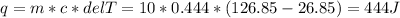
Thus, the heat energy absorbed by iron block is 444 J.
Similarly, the heat energy absorbed by copper block with mass m = 10g, ΔT = 100 °C and c = 0.386 J/g°C is
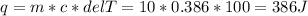
And, the heat energy absorbed by the copper block is 386 J.
Thus, the energy absorbed by iron block is 444 J and the energy absorbed by copper block is 386 J.