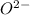The given question is incomplete. The complete question is:
In which compound have electrons been transferred to the oxygen atom from the accompanying atom.
a)
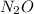
b)
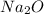
c)

d)

Answer: b)
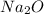
Step-by-step explanation:
An ionic compound is formed when an element completely transfers its valence electron to another element. The element which donates the electron is known as electropositive element or the metal and the element which accepts the electrons is known as electronegative element or non metal.
A covalent compound is formed when an element shares its valence electron with another element. This bond is formed between two non metals.
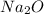 contain ionic bonds as it is made up of sodium metal and oxygen non metal where two sodium atoms loses electrons to form
contain ionic bonds as it is made up of sodium metal and oxygen non metal where two sodium atoms loses electrons to form
 and oxygen gains two electrons to form
and oxygen gains two electrons to form