Answer:
Increase in pressure or concentration and decrease in temperature favors the formation of the liquid and the solute in the equilibrium between a liquid and its vapor and in the equilibrium between a solute and a solvent respectively.
Step-by-step explanation:
In the equilibrium between a liquid and its vapor, as the pressure of the vapor increases it tends to favor the formation of the liquid, hence
Liquid ⇄ Vapor
![Liquid\xrightarrow[]{Decrease \,\, In\, \, Pressure} Vapor](https://img.qammunity.org/2021/formulas/chemistry/high-school/kvsksmuak4ywrabxstjokavy2elrfwjdk3.png)
By Raoult's law we have that the concentration of the components of an ideal mixture is proportional to the mole fraction and vapor pressure
That is
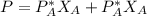
Also increase in temperature favors the formation of the vapor
![Liquid\xrightarrow[]{Increase\,\, In\, \, Temperature} Vapor](https://img.qammunity.org/2021/formulas/chemistry/high-school/r2yfgz2ozamu5r6b964gvta8axo5lbazg2.png)
Similarly the in the equilibrium between a solute and a solvent we have
![Solution\xrightarrow[]{Increase\,\, In\, \, Concentrtion} More \,Solute \, Residue](https://img.qammunity.org/2021/formulas/chemistry/high-school/sigdd72ggolzjl439kt9b1i1bt4jwt8b48.png)
While decrease in concentration results in an excess amount of solvent
So also, increase in the temperature of the solution results in the consumption of excess solute
![Excess \,Solute\,(Oversaturated \,\,solution)\xrightarrow[]{Increase\,\, In\, \, Temperature} Saturated \, solution](https://img.qammunity.org/2021/formulas/chemistry/high-school/nxfrzdxo9cxnaj0zt22fsx2of6lsm2izj3.png)