Answer:
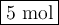
Step-by-step explanation:
1. Gather all the information in one place.
S + 3F₂ ⟶ SF₆
n/mol: 6 15
They have given us the amounts of two reactants and asked us to calculate the amount of product. This is a limiting reactant problem.
2. Calculate the moles of SF₆ you can obtain from each reactant
From S:
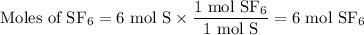
From F₂:
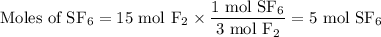
SF₆ is the limiting reactant, because it gives fewer moles ( 5 mol) of SF₆.
The reaction can form 5 mol of SF₆.