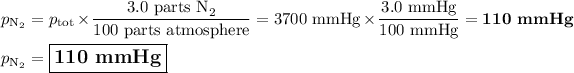Answer:
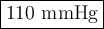
Step-by-step explanation:
We can use Dalton's Law of Partial Pressures:
Each gas in a mixture of gases exerts its pressure separately from the other gases.
In other words, if a gas makes up 3.0 % of the atmosphere, its partial pressure is 3.0 % of the total pressure.