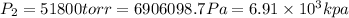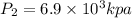Answer:
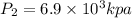
Step-by-step explanation:
Assuming that temperature is constant
According to Boyle's Law, at constant temperature pressure is inversly proportional to the volume and mathematically it can be expressed as:
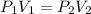 ..........1
..........1
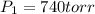
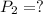
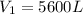
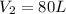
from the first equation after putting all the value
we get,