Complete Question:
When specially prepared Hydrogen atoms with their electrons in the 6f state are placed into a strong uniform magnetic field, the degenerate energy levels split into several levels. This is the so called normal Zeeman effect.
Ignoring the electron spin what is the largest possible energy difference, if the magnetic field is 2.02 Tesla?
Answer:
ΔE = 1.224 * 10⁻²² J
Step-by-step explanation:
In the 6f state, the orbital quantum number, L = 3
The magnetic quantum number,
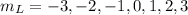
The change in energy due to Zeeman effect is given by:
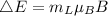
Magnetic field B = 2.02 T
Bohr magnetron,
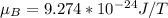
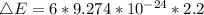
ΔE = 1.224 * 10⁻²² J